Release notes
R&S ® ARB Toolbox PLUS Software Release Notes Version 2.8 © 2014 Rohde & Schwarz GmbH & Co. KG Printed in Germany – Subject to change – Data without tolerance limits is not binding. R&S® is a registered trademark of Rohde & Schwarz GmbH & Co. KG. Trade names are trademarks of the owners. The following abbreviations are used throughout this document
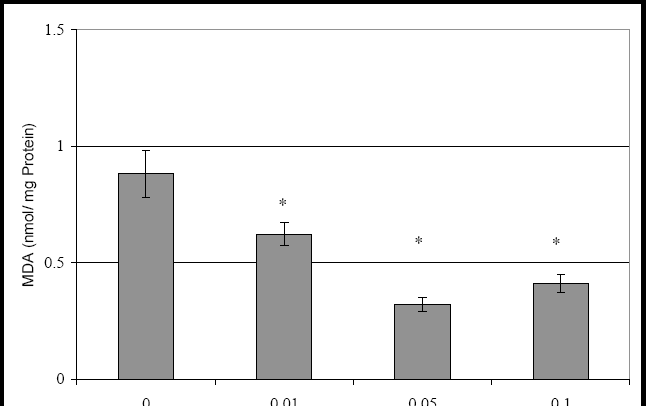

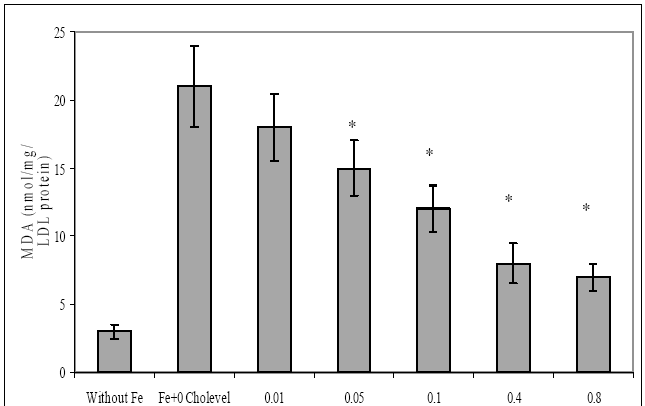
 88 The Open Complementary Medicine Journal, 2009, Volume 1
88 The Open Complementary Medicine Journal, 2009, Volume 1